This guest blog post was written by Dr. David Marshall, a technical writer, and a training developer since 2007. He is also the owner of Neithdos Consulting Services LLC. Dr. Marshall received his DM in Executive Leadership from Colorado Technical University in 2021.
There is a saying that the best form of flattery is when someone copies your work. However, as writers we may take exception, because of the time and effort that we put in. As doctoral students, we are taught to use previous research done by others to fill in the knowledge gap. If we are writing blogs or articles, we may use information found on the internet. This is where we need to give credit by citing the author’s work.
Did you know there are documents that encourage writers to copy your competitor’s work through a policy called Substantial Equivalence Requirement for 510(K) Clearance and from the U.S. Food and Drug Administration (FDA)? These documents are called an Instruction for Use or IFU.
For example, in manufacturing contexts, particularly those involved with production of complex machinery or pharmaceuticals, learning content management systems for manufacturing play a pivotal role in ensuring that accurate and compliant IFUs are readily available, enhancing both safety and usability of products.
In this blog, we will learn what an IFU is, its purpose, how to write the document and the responsibilities of the manufactures that produce IFUs.

What is an Instruction for Use
Instructions for Use or IFUs is information provided by the manufacturer to inform the user of a device’s intended purpose, proper use, and any precautions. IFUs are used mainly in the medical field, specifically for prescription drugs and medical devices.
IFUs are included in all devices including ones that have the smallest modifications which are intended to improve the quality of the product even if the original is still on the market.
IFUs need to include a detailed description of the device including identifying the differences between related products even if it was manufactured by your own company.
According to Code of Federal Regulations Title 21, all medical equipment, devices, and accessories sold for clinical use needs to include an IFU. However, the only exception are Class I and Class IIA if it can be used safely without any instructions. An example of Class I products include bandages, handheld surgical instruments, and nonelectric wheelchairs. Class IIA products include:
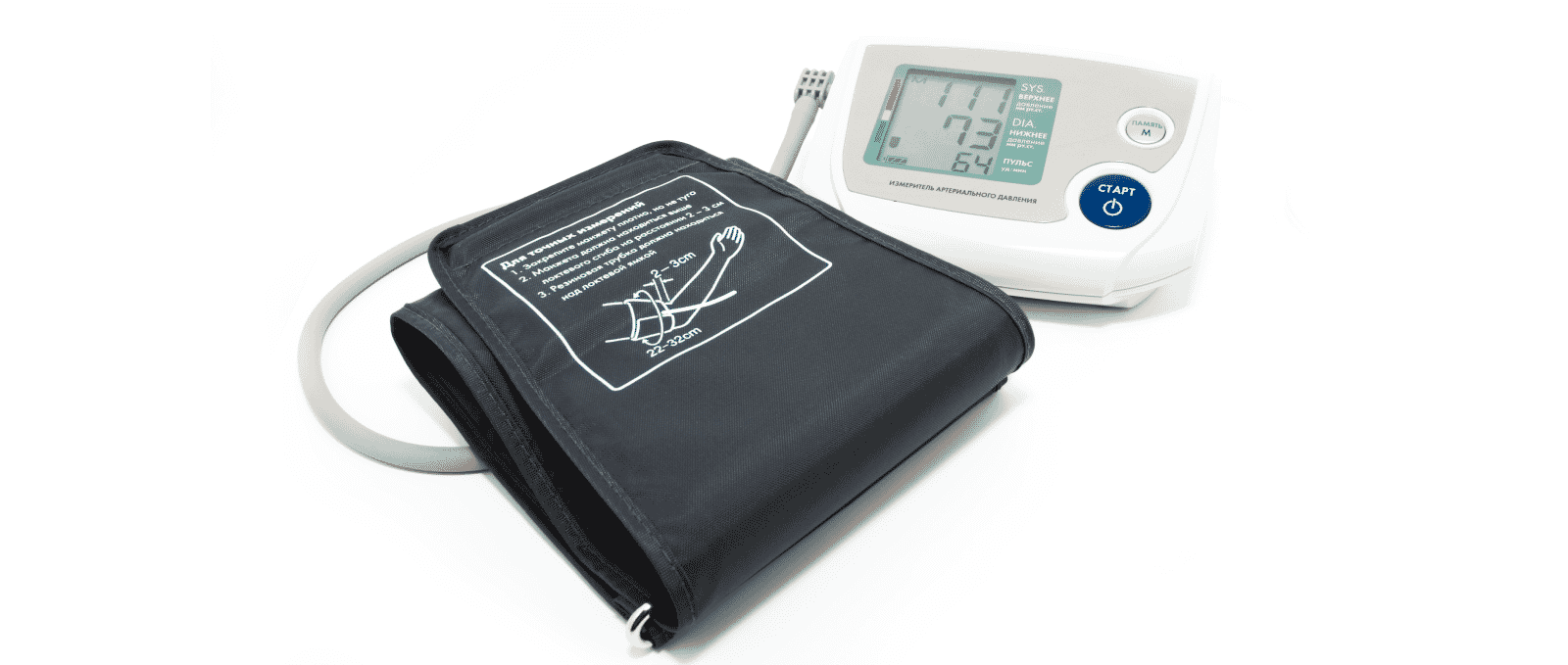
- Blood Pressure Cuffs
- Syringes
- Blood Transfusion Devices
- Powered Wheelchairs
- Contact Lenses
- Software used as Diagnostic Tools
Companies that do not provide IFUs with the product must be able to answer any questions from customers including if the device is safe to use without the documentation.
Note: If hard copies of the IFU are not available, the manufacturer must provide it in electronic form.
Comprising an IFU
All IFUs need to be in a clear, step-by-step instructional hierarchy with headings and subheadings. Chapters, procedural steps, troubleshooting sections, and a glossary add depth and clarity to your composition. Remember, the structure you choose should match the content and the preferences of your user base. Here is an example of how the information should be presented in the following order:
1. Title – “Instructions for Use”
2. Product Title
- Proprietary Name (If any)
- Nonproprietary name
- Dosage Form
- Route of Administration
3. Purpose Statement
- Informs the patient about the content and why it was created.
4. Visual of Drug Product
- Line Drawings
- Simple Illustration
- Photographs
- Ensure each part is clearly identified and labeled.
5. Important Information for Patients
6. Preparation Instructions
7. Administration Instructions
8. Storage Instructions
9. Disposal Instructions
10. Additional Information
It is recommended to use active voice, make the steps short, to the point and use images to illustrate each step. When it comes to crafting instructions, embracing human factors is crucial. Just like choosing the right chew toy for a puppy, understanding your audience is key. Take a moment to get into your users' heads and identify their needs, preferences, and language requirements. Tailor your instructions to their unique characteristics.
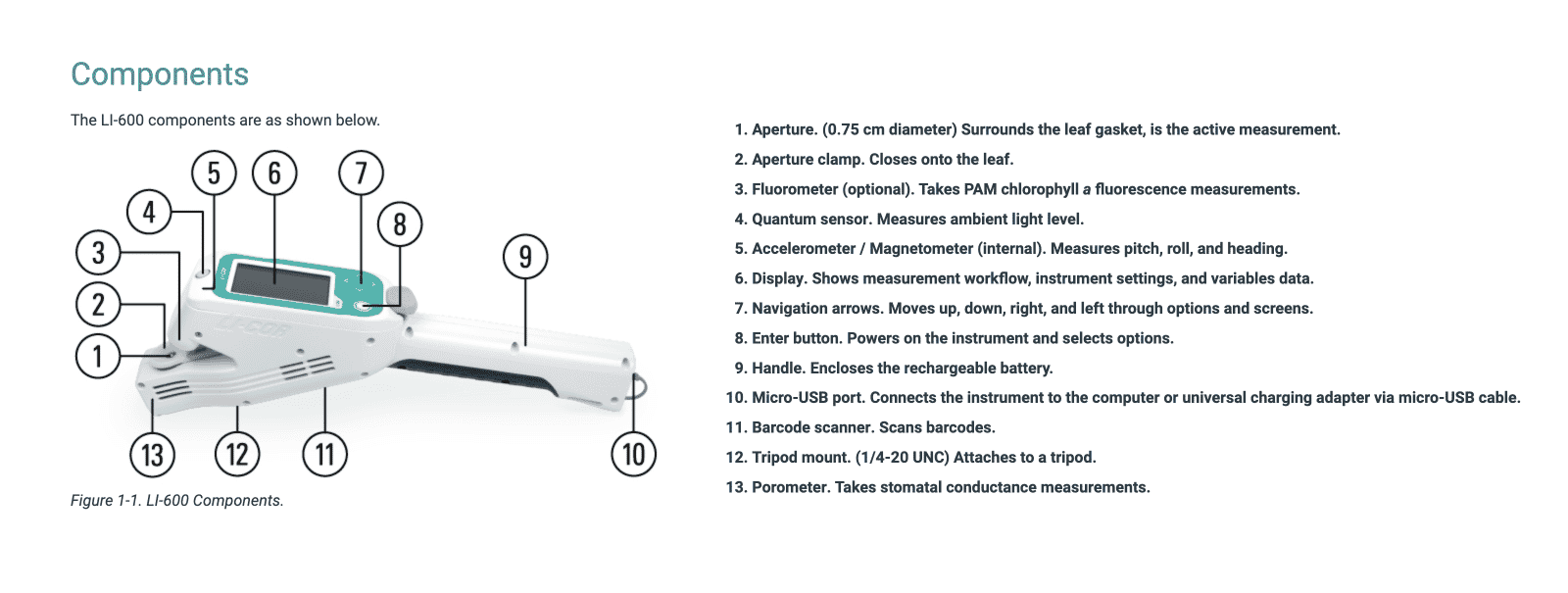
Credit image: Top Nav | LiCOR Technical Support Center | LI-600 Porometer/Fluorometer
Styling & Font
IFUs should be written in sans-serif style fonts such as Verdana and Arial. The font size should be no smaller than 10pt. The only exceptions are:
- Name and place of business of the manufacture, packer, or distributor
- Verbatim statement: This “Instructions for Use” has been approved by the U.S. Food and Drug Administration
- Date of approval or revision of the IFU by the FDA.
In these cases, 8pt font or more sufficed.
Other items to consider:
- INSTRUCTIONS FOR USE should appear in all uppercase letters and in bold.
- Product title, Headings, Step numbers and Figure titles should appear in bold.
For more guidance on drafting an IFU refer to Instructions for Use - Patient Labeling for Human Prescription Drug and Biological Products and Drug-Device and Biological-Device Combination – Content and Format which is available online.
Responsibilities and Liabilities
Manufactures can be held liable for injuries caused by unsafe use of the product due to unclear or misleading instructions to include using erroneous information in translations. To be successful in today’s world, organizations need to create online help documentation to help answer the questions that clients have.
Whether it is instructions for assembling furniture or medical devices and equipment for use by medical professionals, keeping users safe is a top priority. Don't be afraid to sound the alarm when necessary. Warn users about potential hazards and provide clear indications of how to navigate dangerous waters. Whether it's working with chemicals, handling medical equipment, or operating complex machinery, prioritize safety above all. Remember, a single adverse event can be a showstopper, so play it safe and always include comprehensive safety information.
Proofing, Editing and Continuous improvement
After writing your content on a user manual software, take a breather, and then proofread. Hunt down any typos, eliminate confusing sentences or phrases, and fine-tune your wording. A polished and error-free instruction manual will leave a lasting impression on your users.
If possible, encourage users' feedback. Embrace constructive criticism and use it to refine your content. By listening to your audience, you'll stay in tune with their needs and maintain a solid reputation in your industry.
Differences Between a User Manual and IFU
The difference between a user manual and an IFU is that the user manual is a comprehensive reference on how to operate and maintain a product. On the other hand, user manuals may provide design theory and include information found in separate guides and instructions.
As mentioned above, an IFU is a procedure used to accomplish a specific task. These instructions can be contained in a user manual or as a standalone document. A notable example is the user manual is used to operate a medical device, while an IFU focuses on performing tasks on said medical device.
Summary
As a writer, we may be asked to create an IFU if supporting the health industry. These instructions are vital not only to the manufacture’s products but also to our lives. When you have a second just look at any prescription drug, vitamins, blood pressure kit, etc. The directions are IFUs which may save a life and prevent injury to include death.