Maximizing Content Efficiency with DITA XML
DITA XML in the Pharmaceutical and Medical Device Industries
Managing technical documentation for organizations, small and large, can be complex at the best of times, but when you add on having to deliver content from IFUs (Information for Use) to Service, maintenance, and installation manuals while at the same time ensuring you are meeting all regulatory requirements, it then becomes a formidable challenge. Addressing this challenge can be streamlined with a pharmaceutical learning content management system. Such a system enhances content accuracy and regulatory compliance, making it indispensable in the complex ecosystems of pharmaceutical and medical device industries. Precision, compliance, and efficiency are not just goals but can be what breaks the organization. Here, DITA XML emerges not just as a tool but as a strategic ally, offering unparalleled efficiency in managing increasingly intricate content needs.
1. Topic-based Authoring
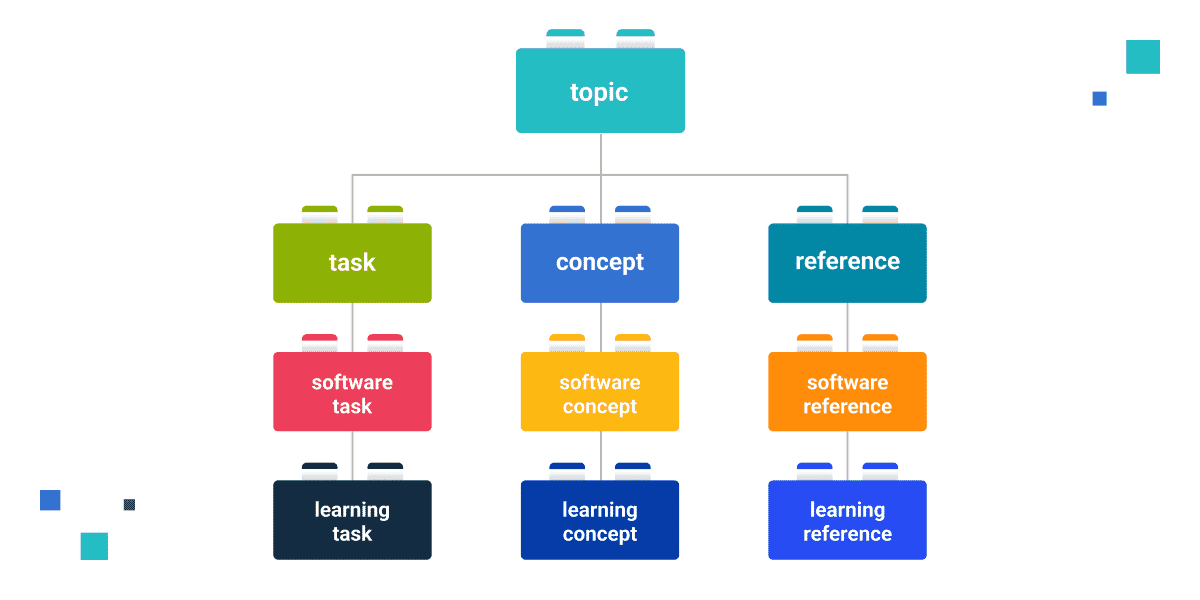
DITA introduces a modular approach to content creation, where each topic represents a distinct idea or chunk of content. These topics are categorized into four main types: Concept, Task, Reference, and Troubleshooting. Concept topics explain ideas, task topics provide step-by-step instructions, reference topics offer detailed specifications, and troubleshooting topics provide steps to resolve issues and problems. This method of categorizing content into concise, self-contained topics enables easier content management and reusability. It allows for the creation of modular, focused, and user-centric documentation, which is essential in conveying complex information clearly. Furthermore, DITA encourages writers to focus on the content of the topic being created. By limiting the elements that can be used in each topic type, the author is "prevented" from straying off the topic by introducing a concept into the task topic they are writing.
2. Enhanced Content Reusability
Let's look at what efficient reuse strategies can do for a pharmaceutical company: the same drug information is needed across multiple documents – from research papers to patient leaflets and instructions for use of the medication. Side effects are common across compounds based on the same molecules; the list goes on. While many help authoring tools offer reuse mechanisms, the DITA XML standard has built-in many different reuse mechanisms that allow for the same content to be used across many functions. Combine this with a powerful CCMS with features such as helping you find reusable content. The combination quickly becomes a game-changer, enabling content reuse and ensuring consistency while saving significant time and resources.
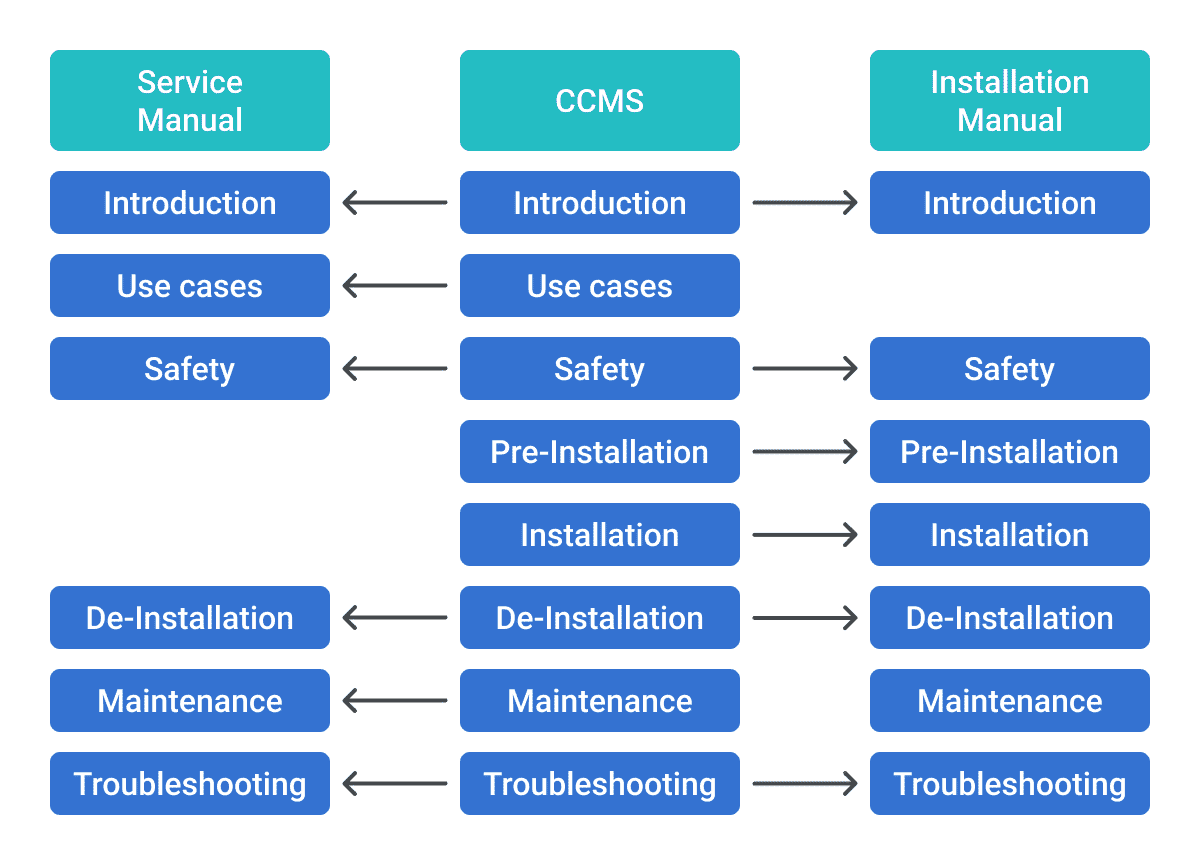
3. Improved Content Consistency
In medical device manufacturing, as in many other industries, the need for uniformity in device manuals across various models is critical. Research into cognitive behavior has shown that readers will find information a lot quicker if the information is presented and structured in the same way every time. The same is true for terminology. DITA XML ensures this consistency, playing a crucial role in maintaining terminology and style – key factors for regulatory compliance, user experience, and patient safety.
4. Easier Localization and Translation
For most manufacturing organizations, the growth of the company is important. Once the local market is saturated, the logical step is to look to sell abroad. To sell globally, an organization must translate or localize their content from the user interface to all the accompanying documents. DITA XML shines in its ability to simplify the translation process for global distribution. Element attributes that add a level of semantic meaning to the content allow the translator to pick the best translation for a term when faced with options. The modular nature of DITA also makes it easier to send smaller units of information for translation. By identifying the specific topics marked for translation and only sending deltas in subsequent translation requests, you reduce costs and improve efficiency. A CCMS with a smooth translation workflow and an ability to connect with Translation Management Systems (TMS) eases a lot of the heavy lifting that comes with creating translation packages. Why continue with out-of-date processes that can introduce defects when a qualified tool integrated with other systems can do it better?
5. Efficient Content Management
Speaking broadly, medical device manufacturers started adopting structured writing in XML, with DITA XML being one of the leading choices in standards. Looking at the volume of content created across the enterprise, it is no real surprise. Managing extensive medical equipment documentation is a daunting enough task, without mentioning managing versions of the documentation, engineering change requests, and more. DITA XML provides a structured approach, making the management of large volumes of content more efficient and less prone to errors. Combined with a CCMS, authors are better able to find, reuse, and otherwise manage the thousands of components that go on to form the product documentation. Because each topic is a self-contained component in the system, it is also easier to version each module, as opposed to the entire monolithic document. Tracking changes to the content is more efficient than having to red-line entire documents manually. You can just point to a list of topics that were impacted, and rest assured, you can prove other changes were made to the document between releases.
6. Seamless Integration with Other Systems
Management product risks, requirements, and defects; legal and regulatory departments using different systems; so many tools, so many departments—trying to keep in line with quality management within the organization can often lead to chaos. Natural silos between the different actors in the development of multiple products mean that information within the organization is hard to find and maintain. By connecting the various systems within an organization to the CCMS, data and information exchange can be facilitated between the different departments. A requirement change in the requirements database can allow an impact assessment to be made in minutes on where and how the documentation needs to be updated.
A compelling use case in manufacturing allows the exchange of information between the DITA XML and the iiRDS standard (intelligent information Request and Delivery Standard). This integration exemplifies how DITA XML can adapt and connect with various systems, enhancing content workflows. OEMs (Original Equipment Manufacturers) can pull the correct information from the CCMS using the metadata to build personalized information sets for their recipients. In this use case, iiRDS acts as an interpreter between the OEM and their customers and can deliver the right personalized information set to each of them.
7. Multi-channel Publishing
DITA XML's ability to support publishing across different platforms and formats is crucial. By creating your content in XML, a markup language that allows for structure with additional ability to contextualize the elements by adding metadata, instructions for publication to different outputs can be understood by the other publication mechanisms. So, the same content can be pushed to deliver the content as PDF HTML files or even pushed to Learning Management Systems to create learning and training materials.
8. Enhanced Collaboration Among Teams
DITA XML fosters improved collaboration between writers, editors, and subject matter experts. The standard encourages the principle of writing for reuse, contributing to a shared pool of information that can be reused across multiple outputs. For editors and SMEs, contributing and reviewing content becomes easier. By using a CCMS, workflows, and version controls, previously approved topics can be identified and even locked from accidental changes during the review cycle. Review workflows can be collaborative, allowing actors in the review to comment and accept changes in real-time. This approach to review and approval can reduce the time it takes to obtain final approval for new and changed content.
9. Scalability for Growing Content Needs
Content and especially documentation volume and variability are significant challenges for growing businesses. Content scalability is built into DITA XML, ensuring that as a business grows, its content management capabilities can grow seamlessly and efficiently. Combine that with an efficient CCMS, and you can increase your content in line with the growth of your product lineup and enterprise size.
Conclusion
DITA XML stands as a robust solution to the unique challenges faced by regulated industries, including medical devices and pharmaceuticals, but it is well suited to manufacturing as well. Its ability to enhance content management, ensure consistency, integrate with various systems, and exchange data, as exemplified in the manufacturing use case with iiRDS, makes it an invaluable asset. Adopting DITA XML goes beyond mere content strategy; it's a step towards more efficient, compliant, and user-focused documentation.
FAQs
How does DITA improve content development?
DITA XML streamlines and simplifies the content development process, ensuring accuracy, consistency, and adaptability across various platforms and formats. As an established standard, DITA includes built-in rules that keep authors on track within a particular topic type. These rules are defined in a file called a DTD (Document Type Definition). DTDs allow for real-time validation of the content being developed by guiding writers in choosing elements that are permitted for a particular topic type. That way your task stays a task without any descriptive conceptual information that is not needed to perform the task.
How can DITA XML enhance collaboration and content sharing across different departments?
DITA XML facilitates a unified content strategy, allowing different departments to collaborate effectively and share content seamlessly, ensuring consistency and accuracy. Content is managed by the repository, helping to maintain versioning, guide against content duplication and more. Different departments can connect to the repository and reuse pre-approved content.
How does DITA XML facilitate collaboration among content teams?
DITA XML fosters a collaborative environment by allowing multiple authors to work on different parts of the content simultaneously. Its modular nature ensures that changes made by one author do not conflict with others, enhancing team efficiency and coherence.
Can DITA XML help in localizing content for global audiences?
Yes, DITA XML is exceptionally beneficial for content localization. Its separation of content from formatting allows for easy adaptation of text for different languages and cultures, making the localization process more streamlined and cost-effective. Combined with a CCMS, translation and localization can only get less expensive as time passes, as only changed content is sent for translation reducing not only the price-per-word cost, but also project management, and the cost of reverifying translations.
Is DITA XML compatible with various content management systems?
DITA XML is designed to be highly compatible with a wide range of content management systems. This compatibility ensures seamless integration and management of content across different platforms, enhancing operational flexibility.
How does DITA XML contribute to cost savings in content production?
By enabling content reuse and efficient management, DITA XML significantly reduces the time and resources needed for content creation and updates. This efficiency translates into considerable cost savings for businesses in their content production processes.
What impact does DITA XML have on the quality of content?
DITA XML enhances content quality by promoting consistency and accuracy. Its structured approach ensures that all pieces of content adhere to predefined standards and formats, resulting in high-quality, professional documentation. Each organization can have their own content strategy and be confident that the combination of the standard and the right CCMS can eradicate many of the “human” errors that can get introduced in content, leaving the content editor with less post-content creation policing to do.
Does DITA XML support multimedia and interactive content?
Yes! DITA XML supports the integration of multimedia elements and interactive content, enabling businesses to create more engaging and dynamic documentation that resonates with modern audiences. Many learning and training organizations have adopted DITA to develop complete educational courses, including tests and revision guides, as the standard allows them to integrate many different multimedia file types.
How does DITA XML adapt to future content trends and technologies?
DITA XML is built with flexibility and scalability in mind, making it well-suited to adapt to evolving content trends and emerging technologies. This future-proof nature ensures that businesses can stay ahead in their content strategies. A fine example of this is the semantic nature of DITA metadata, which means content can be used to train LLMs (Large Language Models) and Chatbots without too much human interaction, as the context can be "read" by machines.