This guest blog post is written by Mohd Sohel Ather, a senior primary software engineer. He focuses on actionable and unique ideas that can be used to grow your business and make a real difference for your customers.
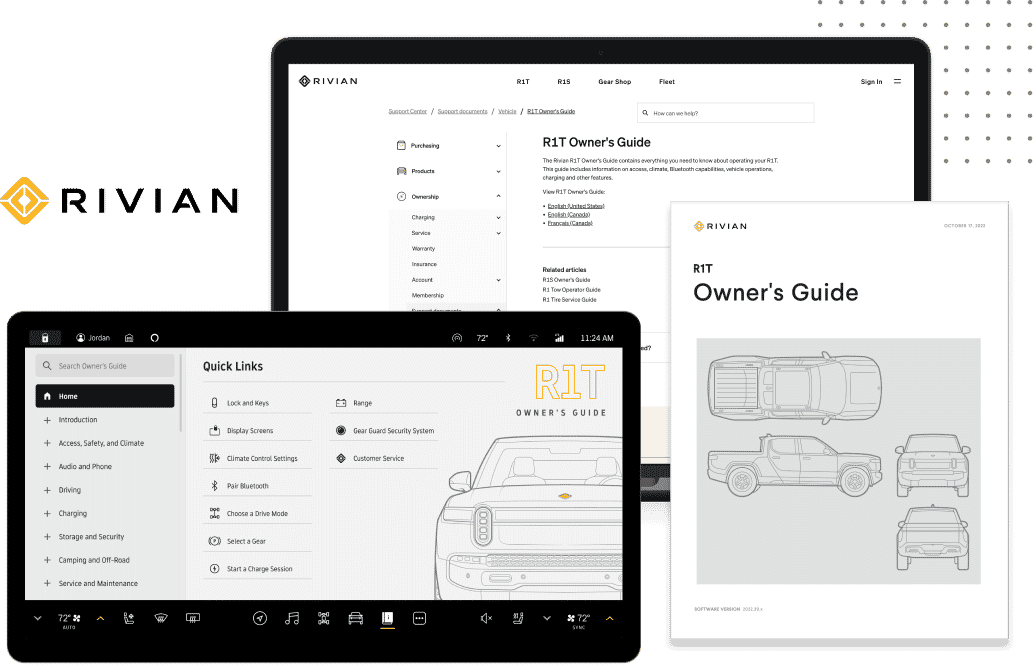
Whether it is used for medical devices or an application in a highly regulated space, good technical documentation processes can’t be neglected. Good documentation practices are mandatory for the pharmaceutical industry, including businesses dealing with medical devices.
Good Documentation Practices, or GDocPs, is the systematic procedure of preparation, reviewing, approving, issuing, recording, storing, and archiving documents. Managing this wealth of documentation effectively requires robust systems. Implementing a healthcare learning content management system can help streamline these processes, ensuring compliance and enhancing efficiency. Read on as we discuss how to navigate around medical device technical documentation.
Learn More: What is Technical Documentation?
Reasons to Implement GDocPs for Medical Devices
The pharmaceutical industry has prioritized GDocP standards for ensuring that all pharmaceutical products meet the industry and regulatory requirements.
Objectives for GDocP can be categorized as:
- A clear mention of all the controlled documents, materials, procedures, and methods that are used for controlling and manufacturing items, along with any other specifications.
- Every person working in the medical sector is guided via the document ensuring what they need to do, how they need to do it, and at what time. To support comprehensive training and guidance, incorporating product training software ensures that all staff are well-prepared and fully understand their roles and responsibilities.
- For every release of the product, the GDocP is necessary because it warrants that the person has the authority to release the product when having the required information. Furthermore, implementing effective employee training software ensures that every team member is proficient and compliant with GDocP standards, further securing the release process.
- The documents also work as a piece of evidence for the product offering traceability of it and also works as a perfect record keeping material for audits. A document management system ensures these records are systematically maintained for quick retrieval and compliance.
- The document can be used for analysis, statistical validation data, and review of related items.
Some Facts and Figures About GDocP
All records that are used for regulatory purposes, which are to be FDA compliant, should comply with the 21 CFR Part 11 (CFR Part 11 regulates Electronic Records; Electronic Signatures). The FDA uses this to maintain the system, product validations, audits, audit trains, documentation, and electronic signatures to process every involved data. In addition, it is used to ensure compliance with the product and its quality standards.
The importance of the FDA and making GDocP mandatory can be understood from research that revealed 80 percent of the inspections done by the FDA in China and India in 2016 failed with data integrity due to a lack of reliable documentation. As a result, the FDA issued warning letters to them.
In the pursuit of maintaining compliance and good medical device technical documentation practices and upholding effective quality management, the FDA has implemented the regulation that every company working in this sector needs to maintain and submit compliance reports following the GDocP to the relevant agencies, such as Good Laboratory Practices (GLP), Current Good Manufacturing Practices (CGMP), and Good Clinical Practices (GCP).
The GDocP process implemented by the FDA also elaborates on the guidelines and best practices about the exchange and interoperability of information, the history of any transaction, and statements about the transactions. This guideline came into existence in 2015, and any company that falls under the sector needs to abide by it.
There have been incidents where the failure to comply with proper GDocP standards or falsifying the evidence can lead to imprisonment and criminal charges.
Hence, it is vital to ensure that any medical device or pharmaceutical company globally implements a proper GDocP as part of a comprehensive quality management system.
Importance of Good Document Practice in Medical Devices?
One should never fail in recording and reporting any manufacturing, production, information and transaction as mentioned below:
- The details about a processing batch
- The way a device has been produced or procured
- The decision about releasing a pharmaceutical product or batch
- Any corrective or Preventive Action (CAPA) taken regarding the product or batch and its evidence
- Reports on any allegation about manufacturing complaints, deviations, and defects
It can be regarded as a failure to abide by the regulatory expectation of GDocP that have been enforced by the likes of WHO, FDA, EMEA, TGA, or Health Canada.
Some benefits of the GDocP in the medical device sector are as follows:
Accuracy of Data
The concept called ALCOA is practiced and followed in GDocP. ALCOA is the acronym for:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
ALCOA offers many benefits, and ensures your content is accurate, reliable, and sustainable.
Keep Track Of Changes
Using Good Documentation Practices and following a standard operating procedure ensures quality assurance and allows one to easily track changes that have occurred in the manufacturing process. It also promotes opportunities to improve the medical device technical documentation process which can be tracked in real-time through methodologies like DITA technical writing.
Maintaining Records
Despite frequent changes in the regulatory requirements, companies need to maintain strict adherence to these guidelines when writing technical documentation to avoid penalties. GDocP helps maintain accurate data and records through effective document management.
A Centralized Database
A centralized database and an efficient content delivery platform are critical in maintaining and managing content. Multiple technical writers, developers, and stakeholders can streamline workflows and adhere to content standards. Maintaining a central database or source of content supports content accuracy, and helps meet mandatory requirements that are essential to the medical device industry.
Final Words GDocPs for Medical Devices
This article gives you a transparent overview of the Good Documentation Practices for medical devices that are necessary because the regulatory requirements demand so much and offer an overall benefit for the company. To get a quality cloud-based project management system that meets all your requirements, contact the right vendor. For more information, explore MadCap as a medical documentation software that can help streamline your documentation processes and ensure compliance.
Accuracy of Data
The concept called ALCOA is practiced and followed in GDocP. ALCOA is the acronym for:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
ALCOA offers many benefits, and ensures your content is accurate, reliable, and sustainable.
Keep Track Of Changes
Using Good Documentation Practices and following a standard operating procedure ensures quality assurance and allows one to easily track changes that have occurred in the manufacturing process. It also promotes opportunities to improve the medical device technical documentation process which can be tracked in real-time through methodologies like DITA technical writing.
Maintaining Records
Despite frequent changes in the regulatory requirements, companies need to maintain strict adherence to these guidelines when writing technical documentation to avoid penalties. GDocP helps maintain accurate data and records.
A Centralized Database
A centralized database is critical to maintaining and managing content. Multiple technical writers, developers, and stakeholders can streamline workflows and adhere to content standards. Maintaining a central database or source of content supports content accuracy, and helps meet mandatory requirements that are essential to the medical device industry.
Final Words
This article gives you a transparent overview of the Good Documentation Practices for medical devices that are necessary because the regulatory requirements demand so much and offer an overall benefit for the company. To get a quality cloud-based project management system that meets all your requirements, contact the right vendor.